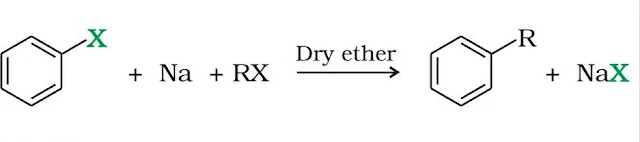Haloalkanes and Haloarenes Class 12 Notes Chemistry Chapter 10
Introduction
In this Unit, you will study the important methods of preparation, physical and chemical properties and uses of organohalogen compounds. Alkyl halides or haloalkanes and aryl halides or haloarenes are organic compounds obtained by replacement of one or more H-atoms of aliphatic and aromatic hydrocarbons respectively by halogen atom(s).
Classification
Haloalkanes and haloarenes may be classified as follows:
I. Based on the Number of Halogen Atoms
(a) Monohaloalkanes and monohaloarenes are compounds containing one halogen atom only, e.g., chloroethane, chlorobenzene etc.
CH3ㅡCH2ㅡCl (Cloroethane)
C6H5Cl (Clorobenzene)
Monohaloalkanes may be further classified as primary (1°), secondary (2°) or tertiary (3°) depending on whether halogen atom is bonded to 1°, 2° or 3° C-atom, e.g.,
(b) Dihaloalkanes and dihaloarenes are compounds containing two halogen atoms. Dihaloalkanes may be further classified as
(i) Gem-dihalides containing two halogen atoms attached to the same C-atom and
(ii) Vicinal dihalides containing two halogen atoms attached to the adjacent C-atoms. Gem-dihalides are also known as alkylidene halides and vicinal dihalides are also known as alkylene halides. e.g.,
(c) Polyhaloalkanes and polyhaloarenes are compounds containing three or more halogen atoms. They may be attached to the same or different C-atoms, e.g.,
CHCl3 (Chloroform), CHBr3 (Bromoform), CHI3 (Iodoform), CCl4 (Carbon tetrachloride)
II. Based on the Number of Halogen Atoms
(a) Allylic halides are compounds containing halogen atom bonded to sp3 hybridised C-atom next to carbon, carbon double bond, e.g.,
CH3ㅡCH=CHㅡCH2ㅡCl {1-Chlorobut-2-ene (1°)}
CH2=CHㅡCH2ㅡCl {3-Chloropropene (1°)}
(b) Benzylic halides are compounds containing halogen atom bonded to sp3 hybridised C-atom next to an aromatic ring. For example
Read also: Alcohols, Phenols and Ethers Chemistry Class 12 Notes Chapter 11
III. Based on the Number of Halogen Atoms
(a) Vinylic halides are compounds containing halogen atom bonded to sp2 hybridised C-atom of an aliphatic compound, e.g.,
(b) Aryl halides are compounds containing halogen atom bonded to sp2 hybridised C-atom of an aromatic ring. For example
IUPAC Nomenclature
Haloalkanes and haloarenes are named as halogen-substituted hydrocarbons in the IUPAC system of nomenclature. The rules of naming such compounds are same as used for hydrocarbons considering halogens as substituents. The common names of these compounds are derived by writing the appropriate name of alkyl or aryl group followed by halide, e.g.,
Methods of Preparation of Haloalkanes
Haloalkanes may be prepared by any one of the following methods.
1. From Alcohols
(a) By reaction with halogen acids: Alcohols react with halogen acids under anhydrous conditions to form haloalkanes. Chloroalkanes are synthesised by treating alcohol with HCl in presence of anhydrous ZnCl2. This is known as Groove’s process.
CH3CH2OH + HCl ⟶ CH3CH2Cl + H2O
(b) By reaction with phosphorus halides:
CH3CH2OH + PCl5 ⟶ CH3CH2Cl + POCl3 + HCl
CH3CH2OH + PCl3 ⟶ CH3CH2Cl + H3PO3
(c) By reaction with thionyl chloride:
CH3CH2OH + SOCl2 ⟶ CH3CH2Cl + SO2 + HCl
Read also: Wave Optics Class 12 Physics Notes Chapter 10
2. From Hydrocarbons
(a) From Alkanes: Alkanes react with halogens in presence of sunlight and/or high temperature to give a mixture of mono and polyhaloalkanes. Monohaloalkane is the major product if alkane is used in excess. If halogen is used in excess, one by one all hydrogens are substituted by halogens.
CH3ㅡCH3 + Cl2 ⟶ CH3ㅡCH2Cl
(b) From Alkenes: Hydrogen halides (HCl, HBr or HI) add to alkenes to form alkyl halides. Addition of hydrogen halide to unsymmetrical alkene follows Markovnikov’s rule with the possibility of rearrangement.
CH3ㅡCH=CH2 + HCl ⟶ CH3ㅡCH(Cl)ㅡCH3
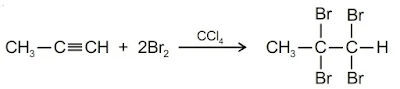
(c) From Alkynes: Alkynes react with Cl2 or Br2 dissolved in CCl4 to give vicinal tetrahalides, e.g.,
3. Halogen exchange
Alkyl iodides are generally prepared by halide exchange in Finkelstein Reaction. In this reaction, alkyl chloride or alkyl bromide is treated with NaI in acetone or methanol to give alkyl iodide in fairly good yield.
CH3CH2Cl + NaI ⟶ CH3CH2I + NaCl
Read also: Conceptual Questions for Class 12 Physics Chapter 10 Wave Optics
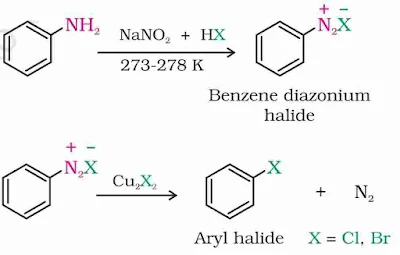
4. From Diazonium Salt
Aromatic primary amine is first converted into diazonium salt by treating it with a mixture of NaNO2 and HCl at 0–5°C. The diazonium salt is used to prepare aryl fluoride by first converting it into diazonium fluoroborate and then heating it. Aryl chloride/bromide is obtained by treating diazonium salt with Cu2Cl2/Cu2Br2 (Sandmeyer reaction) or Cu powder and HCl/HBr (Gatterman reaction).
Physical Properties
Pure alkyl halides are colourless. Many volatile halogen compounds have sweet smell.
Methyl chloride, methyl bromide, ethyl chloride and some chlorofluoromethanes are gases at room temperature. Higher members are liquids or solids.
The haloalkanes are very slightly soluble in water.
Bromo, iodo and polychloro derivatives of hydrocarbons are heavier than water. The density increases with increase in number of carbon atoms, halogen atoms and atomic mass of the halogen atoms
Chemical Properties
1. Reactions of Haloalkanes
The C—X bond of haloalkanes is polar with partial positive charge on carbon and partial negative charge on halogen. Any nucleophile stronger than halide ion can attack at the C-atom due to positive charge causing nucleophilic substitution. Halide ions being weak bases and are good leaving groups, thus haloalkanes undergo elimination reaction with a strong base.
I. Nucleophilic Substitution Reactions
In nucleophilic substitution reactions, the incoming nucleophile having at least one atom with a lone pair of electrons attacks at the carbon atom bonded to halogen.
The nucleophilic substitution proceeds mainly by two different mechanisms as described below:
(a) Substitution nucleophilic bimolecular (SN2)
SN2 is a single step bimolecular reaction in which the incoming nucleophile attacks the C–atom of substrate in a direction opposite to the outgoing nucleophile. The reaction passes through a transition state in which both the incoming and outgoing nucleophiles are bonded to the same C–atom.
(b) Substitution nucleophilic unimolecular (SN1)
SN1 is a two step unimolecular reaction. The first step is the slow ionisation of substrate and is the rate-determining step. The second step is the rapid reaction between the carbocation (formed in the first step) and the nucleophile. SN1 reactions generally proceed in polar protic solvents such as H2O, CH3OH, CH3COOH etc.
RㅡX ⟶ R+ + X- (Step 1)
R+ + Nu- ⟶ RㅡNu (Step 2)
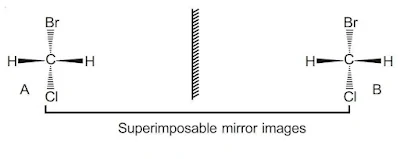
i). Optical Isomerism
When a compound is non super imposable on its mirror image. The compound would be chiral and it will exhibit optical isomerism. All chiral objects rotate plane polarized light.
Chiral and hence X and Y are non-identical. They are enantiomers.
A and B are identical hence X is chiral while A is achiral. X will exhibit optical isomerism.
ii). Chiral Carbon
If all the four valencies of carbon are satisfied by 4 different group the carbon is chiral carbon or asymmetric carbon. Presence of chiral carbon never ensure the molecule is optically active.
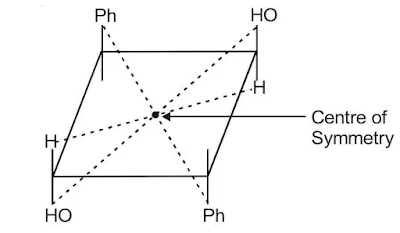
iii). Elements of Symmetry
Elements of symmetry offer a simple device to decide whether a molecule is chiral or achiral, i.e., whether the molecule is superimposable or non-superimposable on its mirror image. When a molecule lacks all element of symmetry it is chiral.
(i) Plane of symmetry (σ): Any imaginary plane which divides the full molecule into two halves which are mirror images of each other.
(ii) Centre of inversion (i): Any imaginary point, through which when inversion will be carried out it will repeat equivalent configuration.
II. Elimination Reactions
Haloalkanes having β-hydrogen atom when heated in presence of alc. KOH undergo dehydrohalogenation forming alkene.
CH3CH2(Cl)CH3 ⟶ CH3CH=CH2 + HCl
III. Reaction with Metals
Haloalkanes react with metals in presence of dry ether forming organometallic compounds. The reactivity of organometallic compounds depends on electropositivity of the metal.
CH3Cl + Mg ⟶ CH3MgCl
when haloalkane is treated with sodium in presence of dry ether, an alkane having twice the number of C-atom as present in haloalkane is formed (Wurtz Reaction).
2CH3Cl + 2Na ⟶ C2H6 + 2NaCl
IV. Reduction of Haloalkanes
Haloalkanes can be reduced to alkanes by number of reducing agents like (i) Zn/CH3COOH, (ii) Zn/HCl, (iii) Zn/NaOH, (iv) Zn-Cu couple/ethanol, (v) LiAlH4, (vi) NaBH4 etc.
RㅡX ⟶ RㅡH
2. Reactions of Haloarenes
I. Nucleophilic Substitution Reactions
Haloarenes are very much less reactive than haloalkanes towards nucleophilic substitution reactions. In haloarenes the lone pair of electrons on halogen atom is delocalised with the π-electrons of benzene ring. It acquires partial double bond character in carbon halogen bond due to resonance. Therefore, it is more difficult to break carbon-halogen bond in haloarenes than in haloalkanes.
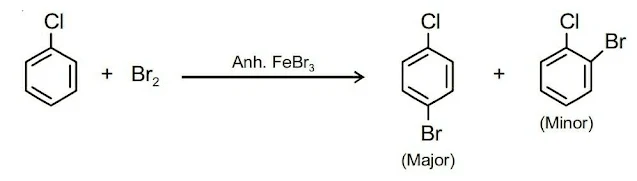
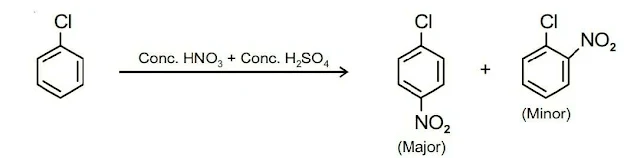
II. Electrophilic Substitution Reactions
Haloarenes are somewhat less reactive than benzene towards electrophilic substitution reactions of the benzene ring due to –I effect of halogen atom which decreases the electron density of benzene. Haloarenes undergo halogenation, nitration, sulphonation, and Friedel-Crafts reactions.
(i). Halogenation (ii). Nitration (iii). Sulphonation (iv). Friedel-Crafts reactionIII. Reaction with Metals
Fittig reaction: Haloarenes when treated with sodium in presence of dry ether react in a manner simillar to haloalkanes to give diarenes. This is known as Fittig reaction.
Wurtz-Fittig reaction: When a mixture of haloalkane and haloarene is treated with sodium under identical conditions we get alkyl arene.
Polyhalogen Compound
Carbon compounds containing two or more than two halogen atoms are generally referred to as polyhalogen compounds. A compound containing two halogen atoms is called dihalide and if it contains three halogen atoms, it is called trihalide and so on.
1. Dichloromethane (Methylene chloride)
Dichloromethane is widely used as a solvent as a paint remover and as a process solvent in the manufacture of drugs. It is also used as a metal cleaning and finishing solvent. Methylene chloride harms the human central nervous system. Higher levels of methylene chloride in air cause dizziness, nausea, tingling and numbness in the fingers and toes. Direct contact with the eyes can burn the cornea.
CH3Cl + Cl2 ⟶ CH2Cl2 + HCl
2. Trichloromethane (Chloroform)
Chloroform is employed as a solvent for fats, alkaloids, iodine and other substances. The major use of chloroform today is in the production of the freon refrigerant R-22. It was once used as a general anaesthetic in surgery. Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is therefore stored in closed dark coloured bottles completely filled so that air is kept out.
CCl4 + H2 ⟶ CHCl3 + HCl
3. Triiodomethane (Iodoform)
It was used earlier as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself.
CH3CH2OH + 4I2 + 6NaOH ⟶ CHI3 + HCOONa + 5NaI + 5H2O
4. Tetrachloromethane (Carbon tetrachloride)
It is produced in large quantities for use in the manufacture of refrigerants and propellants for aerosol cans. It is also used as feedstock in the synthesis of chlorofluorocarbons and other chemicals, pharmaceutical manufacturing, and general solvent use. Depletion of the ozone layer is believed to increase human exposure to ultraviolet rays, leading to increased skin cancer, eye diseases and disorders, and possible disruption of the immune system.
CHCl3 + Cl2 ⟶ CCl4 + HCl
5. Freons
The chlorofluorocarbon compounds of methane and ethane are collectively known as freons. They are extremely stable, unreactive, non-toxic, non-corrosive and easily liquefiable gases. Freon 12 (CCl2F2) is one of the most common freons in industrial use. It is manufactured from tetrachloromethane by Swarts reaction.
CCl4 + 2HF ⟶ CCl2F2 + 2HCl
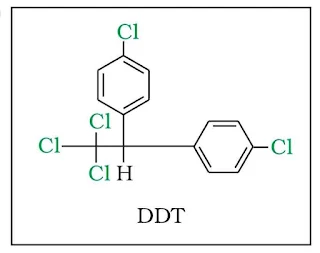
6. p,p’-Dichlorodiphenyltrichloroethane (DDT)
DDT is a colorless, tasteless, and almost odorless crystalline chemical compound. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-born diseases malaria and typhus among civilians and troops.
Summary
Alkyl/Aryl halides may be classified as mono, di, or polyhalogen (tri-, tetra-, etc.) compounds depending on whether they contain one, two or more halogen atoms in their structures.
Since halogen atoms are more electronegative than carbon, the carbon-halogen bond of alkyl halide is polarised.
Alkyl halides are prepared by the free radical halogenation of alkanes, addition of halogen acids to alkenes, replacement of –OH group of alcohols with halogens using phosphorus halides, thionyl chloride or halogen acids.
Aryl halides are prepared by electrophilic substitution to arenes.
Fluorides and iodides are best prepared by halogen exchange method.
The boiling points of organohalogen compounds are comparatively higher than the corresponding hydrocarbons because of strong dipole-dipole and van der Waals forces of attraction.
Organohalogen compounds are slightly soluble in water but completely soluble in organic solvents.
The polarity of carbon-halogen bond of alkyl halides is responsible for their nucleophilic substitution, elimination and their reaction with metal atoms to form organometallic compounds.
Nucleophilic substitution reactions are categorised into SN1 and SN2 on the basis of their kinetic properties.
Chirality has a profound role in understanding the reaction mechanisms of SN1 and SN2 reactions.
SN2 reactions of chiral alkyl halides are characterised by the inversion of configuration while SN1 reactions are characterised by racemisation.
A number of polyhalogen compounds e.g., dichloromethane, chloroform, iodoform, carbon tetrachloride, freon and DDT have many industrial applications.
These compounds cannot be easily decomposed and even cause depletion of ozone layer and are proving environmental hazards.