The d and f-Block Elements Class 12 Notes Chemistry Chapter 8
Introduction
In this chapter, we are going to study the usual and unusual properties of d-block element. Classification of elements in blocks is based on their characteristic properties. During filling of electrons, the last electron decides the block of the element. d-block contains 3 to 12 group. A number of compounds of this block elements are important catalyst in industry.
Position in the Periodic Table
The d-block of the periodic table contains the elements of group 3-12 in which the d orbitals are progressively filled in each of the four long periods. The name “transition” given to the element of d-block is only because of their position between s and p-block elements. There are four series of the transition metals 3d series (Sc to Zn), 4d series (Y to Cd) and 5d (La, Hf to Hg) and 6d series (AC, unq to Uub).
Definition of Transition Element
A transition element is defined as the one which has incompletely filled d orbitals in its ground state or in any one of its oxidation state. Zn, Cd and Hg are not typical transition elements because they have full d10 configuration in their ground state as well as in their common oxidation state.
However, being the last members of three transition series, their chemistry is studied along with the chemistry of transition metals.
Electronic Configurations of the d-Block Elements
General E.C. of the d-block element is as
For 3d series 3d1–10 4s1–2
For 4d series 4d1–10 5s1–2
For 5d series 5d1–10 6s1–2
For 6d series 6d1–10 7s1–2
Overall (n–1) d1–10 ns1–2
The d orbitals of the transition element project to the periphery of an atom more than the other orbitals (s and p). Hence, they are more influenced by the surrounding as well as affecting the atoms or molecules surrounding them.
Read also: Coordination Compounds Chemistry Class 12 Notes Chapter 9
General Properties of the Transition Elements
1. Physical Properties
Nearly all the transition elements display metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre.
The transition metals (with the exception of Zn, Cd, Hg) are very much harder and have low volatility. Their melting points and boiling points are high.
In any row the melting points of these metals rise to maximum at d5 except for anamolous behaviour of Mn and Tc and fall regularly as the atomic number increases. They have high enthalpies of atomisation.
In general greater the number of valence electrons, stronger is the resultant bonding. Since the enthalpy of atomisation is an important factor in determining the standard electrode potential of a metal, metals with very high enthalpy of atomisation i.e., very high boiling point tend to be noble in their reactions.
2. Atomic and Ionic size
In general, ions of the same charge in given series show progressive decrease in radius with increasing atomic number. This is because the new electron enters a d orbital each time the nuclear charge increases by unity and shielding effect of d electron is not effective hence net electrostatic attraction between the nuclear charge and outer most electron increases and the ionic radius decreases.
The filling of 4f before 5d orbitals results in regular decrease in atomic radii called Lanthanoid contraction which essentially compensates for the expected increase in atomic size with increasing atomic number.
3. Density
The decrease in metallic radius coupled with increase in mass results in a general increase in the density of these elements. Thus from Ti to Cu the significant increase in density may be noted.
4. Ionisation Enthalpy
Due to increase in nuclear charge which accompanies the filling of the inner d orbitals there is an increase in ionisation enthalpy along each series of the transition elements from left to right. However many small variations occur.
5. Oxidation State
One of the notable feature of a transition element is the great variety of oxidation states. The elements which give the greatest number of oxidation states occur in or near the middle of series. Mn exhibits all oxidation states from +2 to +7.
6. Chemical Reactivity
Transition metals vary widely in their chemical reactivity. Many of them are sufficiently electropositive to dissolve in mineral acid although a few are noble, it means these do not react with single acid. The metal of 3d series except Cu are relatively more reactive and are oxidised by 1M H+ though the actual rate at which these metals react with oxidising agents like hydrogen ion (H+) is some time slow.
7. Magnetic Properties
When magnetic field is applied to substance. Mainly two types of magnetic behaviour are observed: diamagnetism and paramagnetism. Diamagnetic substance contain no unpaired electron and repelled by magnet while paramagnetic substances are attracted and contain one or more unpaired electron. The substance which are strongly attracted by magnet are said to be ferromagnetic. Paramagnetism arises due to presence of unpaired electrons, each such electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum.
`μ=\sqrt{n(n+2)}` BM
where n is number of unpaired electron and BM–Bohr magneton.
Read also: Electromagnetic Waves Class 12 Physics Notes Chapter 8
8. Formation of Coloured Ions
When an electron from a lower energy d orbital is excited to higher energy d orbital, the energy of excitation corresponds to the frequency of light absorbed. This frequency absorbed generally lies in the visible region. The colour observed corresponds to the complementary colour of light absorbed. The frequency of light that is absorbed is determined by (i) Nature of ligand, (ii) Size of metal ion and (iii) Oxidation state of metal.
9. Formation of Complex Compound
Due to smaller size of the metal ions high ionic charges and the availability of d orbitals for bond formation, transition elements form a number of complex compounds. Example of some complex compounds/ions are:
[Fe(CN)6]3–, [Cu(NH3)4]2+, [Mn(H2O)4]2+, [CuCl4]2-
10. Catalytic Properties
The transition metals and their compounds are known for their catalytic activity. This activity is ascribed to them due to their ability
- To adopt multiple oxidation states
- To form complexes
Catalyst at a solid surface involve the formation of bonds between reactant molecules and atoms of the surface of catalyst. This has the effect of increasing the concentration of the reactants at the catalyst surface and also weakening of the bonds in the reacting molecules. Also because transition metal ions can change their oxidation states, they become more effective as catalyst.
Read also: Conceptual Questions for Class 12 Physics Chapter 8 Electromagnetic Waves
11. Formation of Interstitial Compounds
Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattice of metals. These are usually non stochiometeric and are neither typically ionic nor covalent. For example, TiC, Mn4N, Fe3H, VH0.56 and TiH1.7 etc.
The principle physical and chemical characteristics of these compounds are :
- They have high melting point, higher than those of pure metal.
- These are very hard.
- They retain metallic conductivity.
- They are chemically inert.
12. Alloy Formation
Alloy may be homogeneous solid solution in which the atoms of one metal are distributed randomly among the atoms of the other. Such alloys are formed by atoms with metallic radii that are within about 15 percent of each other. Because of similar radii and other characterstics of transition metals alloys are readily formed by these metals. The alloys so formed are hard and have often high melting point. The best known are ferrous alloys. Cr, V, W, Mo and Mn are used for the production of a veriety of steels and stainless steel.
Preparation and Properties of the Compounds of d-Block Elements
(1) Potassium permanganate (KMnO4)
Potassium permanganate is prepared from pyrolusite (MnO2) involving the following steps:
(i) Conversion of pyrolusite (MnO2) into potassium manganate: Powdered pyrolusite is fused with KOH in the presence of air or an oxidising agent like KNO3, or KClO3 to give green coloured potassium manganate.
2MnO2 + 4KOH + O2 ⟶ 2K2MnO4 + 2H2O
(ii) Oxidation by chlorine
2K2MnO4 + Cl2 ⟶ 2KMnO4 + 2KCl
Properties of Potassium Permanganate
Potassium permanganate forms dark purple needle like crystals with a metallic lusture. Its m.p. is 523 K. It is moderately soluble in water giving a purple solution.
On heating, potassium permanganate changes into manganate and oxygen gas is evolved.
2KMnO4 ⟶ K2MnO4 + MnO2 + O2
On heating with alkalies, potassium permanganate changes into manganate and oxygen gas is evolved.
4KMnO4 + 4KOH ⟶ 4K2MnO4 + 2H2O + O2
Oxidising agent: KMnO4 is a powerful oxidising agent in acidic, neutral and alkaline medium. In acidic medium:
2KMnO4 + 3H2SO4 ⟶ K2SO4 + 2MnSO4 + 3H2O + 5[O]
Structure of manganate and permanganate ion
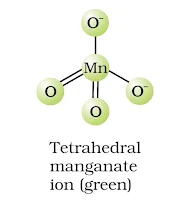
(a) MnO42– ion-tetrahedral structure.
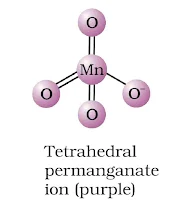
(a) MnO4– ion-tetrahedral structure.
(2) Potassium dichromate, K2Cr2O7
It is the most important compound of chromium. K2Cr2O7 is prepared from chromite ore (FeO.Cr2O3). The various steps involved are:
(i) Preparation of sodium chromate: The powdered ore is heated with sodium hydroxide in the presence of air, sodium chromate is formed and CO2 is given out.
4FeCr2O4 + 16NaOH + 7O2 ⟶ 8Na2CrO4 + 2Fe2O3 + 8H2O.
(ii) Conversion of sodium chromate into sodium dichromate: The solution of sodium chromate is treated with dil. H2SO4 to get sodium dichromate, Na2Cr2O7.
2Na2CrO4 + H2SO4 ⟶ Na2Cr2O7 + Na2SO4 + H2O
(iii) Conversion of sodium dichromate into potassium dichromate: Sodium dichromate is filtered and then treated with dil. H2SO4. Being less soluble, sodium sulphate separates out. Then hot concentrated solution is cooled when red crystals of sodium dichromate separates out on standing and then a hot concentrated solution of sodium dichromate is treated with potassium chloride to get potassium dichromate.
Na2Cr2O7 + 2KCl ⟶ K2Cr2O7 + 2NaCl
Properties
- It is an orange crystalline solid with melting point 670 K.
- It is appreciably soluble in hot water but moderately soluble in cold water.
- On heating K2Cr2O7 decomposes to give potassium chromate and chromic oxide.
4K2Cr2O7 ⟶ 4K2CrO4 + 2Cr2O3 + 3O2
(d) Chromyl chloride test: When a mixture of metal chloride is heated with K2Cr2O7 and conc. H2SO4 acid, orange red fumes of chromyl chloride are formed.
K2Cr2O7 + 4NaCl + 6H2SO4 ⟶ 2KHSO4 + 4NaHSO4 + 2CrO2Cl2 + 3H2O
(e) Reaction with H2O2: Acidified solution of dichromate ions form a deep blue colour with H2O2 due to the formation of [CrO(O2)2].
Cr2O72– + 4H2O2 + 2H+ ⟶ 2CrO5 +5H2O
Uses of K2Cr2O7
- For the volumetric estimation of ferrous salts, iodides and sulphides.
- For the preparation of other chromium compounds such as chrome alum, chrome yellow etc.
- Used in chrome tanning in leather industry.
- Used as an oxidising agent.
Structure of chromate and dichromate ion
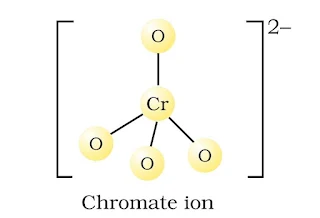
(a) CrO42– ion-tetrahedral structure.
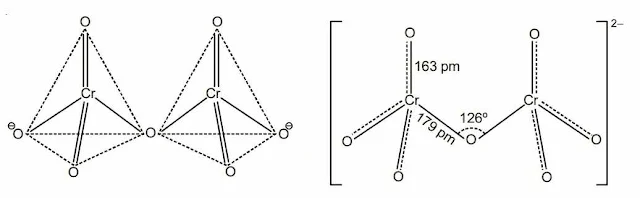
(b) Cr2O72– ion-two tetrahedra sharing one oxygen atom at one corner.
The Inner Transition Elements
The f block consists of two series lanthanoids and actinoids. Lanthanum closely resembles the lanthanoids, it is usually included in any discussion of the lanthanoids for which the general symbol Ln is often used. Similarly a discussion of the actinoids includes actinium besides the fourteen elements constituting the series.
Lanthanoids resemble one another more closely than do the members of ordinary transition elements in any series. They have only one stable oxidation state and their chemistry provides an excellent opportunity to examine the effect of small size and nuclear charge along a series of otherwise similar elements.
The Lanthanoids
1. Electronic Configuration
It may be noted that the atoms of these elements have electronic configuration as 6s2 5d0 or 1 4f1–14.
2. Atomic Size and Ionic Size
Decrease in size from La to Lu is a unique feature in chemistry of lanthanoids. This is due to lanthanoid contraction. The decrease in atomic radii is not quite regular as it is regular in M3+ ion. This contraction is similar to that observed in an ordinary transition series and is attributed to same cause, the imperfect shielding of one electron by another in the same sub-shell.
3. Oxidation state
In the lanthanoids, La(II) and Ln(III) compounds are predominant. However some element may show oxidation state of III and IV. Pr, Nd, Tb and Dy can exhibit +4 O.S. only in MO2 oxide.
4. General Characterstics
- All the lanthanoids are silvery white soft metals and tarnish rapidly.
- Hardness increases with increasing atomic number, samarium (Sm) being steel hard.
- These are good conductor of electricity.
- Density and other properties change smoothly except for Eu and Yb and occasionally for Sm and Tm.
- Many trivalent lanthanoid ions are coloured both in solid state and in aq. solution. Colour of these ions may be attributed to the presence of f electrons.
5. Reaction
6. Uses
- Best use of the lanthanoids is for the production of alloy steels for plate and pipe.
- A well known Mischmetal consists of a lanthanoid metal (~95%) and iron (~5%) and traces of S, C, Ca and Al. It is used in Mg-based alloy to form bullets.
- Mixed oxide of lanthanoids are employed as catalysts in petroleum cracking.
- Some individual lanthanoid oxide are used in television screen and similar florescing surfaces.
The Actinoids
Actionid include the fourteen elements from Th to Lr. The name symbol and some properties of these element are shown as
The actinoids are radioactive elements and the earlier members have relatively long half life, the latter ones have half life values ranging from a day to 3 minute for Lawrencium (Z-103).
1. Electronic Configuration
General electronic configuration of all elements can be consulted from table. The fourteen electrons are formally added to 5f though not in thorium (Z-90) but from Pa onward the 5f orbitals are complete at element 103. The irregularities in the electronic configurations of the actinoids like those in the Lanthanoids are related to the stabilities of the f0, f7, f14 occupancies of the 5f orbitals.
2. Atomic Size and Ionic Size
There is gradual decrease in the size of atoms or M3+ ions across the series. This is referred to as actinoid contraction which is greater than lanthanoid contraction.
3. Oxidation state
There is greater range of oxidation states which is in part attributed to the fact that the 5f, 6d and 7s level are of comparable energies. The elements, in first half of actinoids frequently exhibit higher oxidation states. However, +3 and +4 ions tend to hydrolyse.
4. General Characterstics
- All are silvery in appearance but display a variety of structure. This is due to irregularity in metallic radii.
- These are highly reactive metal.
- HCl react with all actinoids.
- Most of them are slightly affected by HNO3 owing to the formation of protective oxide layer. However, alkalies have no action.
- Magnetic properties of the actinoids are more complex than those of lanthanoid.
- IE1 of early actinoids are lesser than early Lanthanoid because 5f orbitals are deeply burried.
- Actinoids compound or their ions are coloured most probably due to charge transfer or f-f transition.
Some Applications of d & f-Block Elements
- Iron and steels are the most important construction material. Their production is based on reduction of iron oxides, the removal of impurities and the addition of carbon and alloying metals like Cr, Mn and Ni.
- Some compounds are manufactured for special purpose such as TiO for the pigment industry and MnO2 for use in dry cell along with use of Zn.
- Many of the elements or compounds of this block elements are important catalyst.
- AgBr is an important chemical used in photography. Along with AgBr, Ag and AgI can also be used.
- Some compounds like MnO4–, CrO42– are good oxidising agents.
Summary
Transition element: It is defined as one which has incompletely filled d orbitals in its ground state or in any one of its oxidation states.
General E.C. of d-block : (n – 1)d1–10 ns0–2
Melting point increases up to middle and then decreases.
Hg exists in liquid state at room temperature.
Ionisation energy increases from left to right but trend is not regular. Successive ionisation enthalpies do not increase as steeply as the main group elements with increasing atomic number.
These elements show variable oxidation states.
Generally these elements and their compounds are coloured.
Due to the presence of unpaired electrons, these are generally paramagnetic.
KMnO4 and K2Cr2O7 are good oxidising agents.
KMnO4 is prepared from MnO2.
In f-block two series are present
(i) Lanthanoid
(ii) Actinoid
All actinoids are radioactive while in lanthanoid only Pm is radioactive.
For Lanthanoid +3 is most common oxidation state.
Decrease in size due to poor screening of 4f orbitals is known as lanthanoid contraction.
Generally lanthanoids and actinoids or their compounds are coloured.
Lanthanoids have little tendency to form complex.
Actinoids have more tendency to form complex.