Organic Chemistry Some Basic Principles and Techniques Class 11 Notes Chemistry Chapter 12
Introduction
In p-Block chapter you have learnt that the element carbon has the unique property i.e., self-linking property or catenation due to which carbon can form covalent bonds with other carbon atoms. Carbon can form covalent bonds with other elements also such as hydrogen, oxygen, nitrogen, sulphur, phosphorus and halogens.
In this chapter, we shall discuss some basic principles and techniques of analysis needed for understanding the formation and properties of organic compounds. Organic compounds are essential for existence and maintenance of life on earth. These include complex molecules like (DNA) which carry genetic information and proteins which is building blocks of life. Organic compounds also play an important role in material used in daily life such as cloths, fuel, dyes, and medicines etc.
Structural Representations of Organic Compounds
(i). Structural Formulas
The lewis structures can be simplified by representing the two electron covalent bonds by a dash (–). In this representation, a single bond is represented by a single dash (–), a double bond by a double dash (=) and a triple bond by a triple dash (≡). The lone pair on an atom may or may not be shown. This representation is called structural formula.
(ii). Condensed Formulas
In this formula, the arrangement of atoms are shown but the bonds between may be omitted and the number of identical groups attached to an atom are indicated by a subscript.
(iii). Bond Line Formulas
In this representation, the carbon and hydrogen atoms are not shown and the lines between carbon-carbon bonds are shown in a zig-zag manner.
Read also: Hydrocarbons Class 11 Notes Chemistry Chapter 13
In cyclic compounds, the bond-line formulas may be given as follows :
Three-dimensional representation of organic molecules
The three-dimensional (3-D) structure of organic molecules can be represented on paper by using certain conventions. In these formulae, the thick solid (or heavy) line or the solid wedge indicates a bond lying above the plane of the paper and projecting towards the observer while a dashed wedge is used to represent a bond lying below the plane of the paper and projecting away from the observer.
Read also: Nuclei Class 12 Physics Notes Chapter 13
Classification of Organic Compounds
On the basis of their structures, organic compounds are broadly classified as follows :
Open Chain Compounds
These compounds contain open chains of carbon atoms in their molecules. The carbon chains may be either straight chains or branched chains. They are also called aliphatic compounds.
Closed Chain or Ring Compounds
These compounds contain chains or rings of atoms in their molecules.
Alicyclic Compounds : These compounds contain a ring of three or more carbon atoms in them. They resemble aliphatic compounds in many of their properties.
Read also: Conceptual Questions for Class 11 Physics Chapter 12 Thermodynamics
Aromatic Compounds : These have a cyclic system containing at last one benzene ring. The parent member of the family is called benzene. Benzene has a homocyclic hexagonal ring of six carbon atoms with three double bonds in the alternate positions.
Heterocyclic Compounds : In these compounds, the ring contains one or more atoms of either nitrogen, oxygen or sulphur in addition to carbon atoms. The atom other than carbon (such as N, O, S) present in the ring is called hetero atoms.
Functional Groups : An atom or group of atoms which largely determines the properties of the organic compounds particularly the chemical properties.
Homologous Series : Homologous series may be defined as “a series of similarly constituted compounds in which the members possess the same functional group and have similar chemical characteristics”. The two consecutive members differ in their molecular formula by –CH2– group.
1. CH3OH - Methyl alcohol
2. C2H5OH - Ethyl alcohol
3. C3H7OH - Propyl alcohol
4. C4H9OH - Butyl alcohol)
5. C5H11OH - Pentyl alcohol
6. C6H13OH - Hexyl alcohol
Nomenclature of Organic Compounds
The term ‘nomenclature’ means the system of naming of organic compounds. There are two systems of nomenclature:
(i) Trivial or Common System
In this nomenclature, the names of organic compounds were assigned based on their source of origin or certain properties. For instance, citric acid got its name from the source (citrus fruits) from which it was first isolated. Formic acid was named so as it was first obtained from red ant. In Latin, ant word is formica.
(ii) IUPAC System of Nomenclature
A systematic method of naming has been developed and is known as the IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature. In this systematic nomenclature, the names are correlated with the structure such that the reader or listener can deduce the structure from the name.
A. Nomenclature of Alkanes
(i). Straight Chain Hydrocarbons: The names of straight chain hydrocarbons consist of word root and primary suffix. The primary suffix for alkanes is ‘ane’.
The IUPAC names of some unbranched saturated hydrocarbons (Carbon-Carbon single bond) are given below.
(ii) Branched Chain Hydrocarbons: In branched chain hydrocarbons, small side chains of carbon atoms are attached to the main carbon parent chain. These side chains are called as alkyl groups and are prefixed t the name of parent alkane. Alkyl groups are derived from alkane by removal of one hydrogen atom so have general formula CnH2n+1 and represented by –R. An alkyl group is named by replacing ‘ane’ of the alkane to ‘yl’.
B. Branched Chain Hydrocarbons:
By following certain rules the branched chain hydrocarbons can be named without any difficulty:
(i) Longest Chain Rule: The initial step of nomenclature is to identify the longest carbon chain which is then called as parent chain.
(ii) Lowest Number Rule: The numbering of the parent chain is done in such a way that the substituents attached to the parent chain should get the lowest possible position.
(iii) Alphabetical Order of the Side Chain: The names of the alkyl group are prefixed before the nam of the parent chain. The position of the alkyl group is indicated by carefully numbering the parent chain Moreover care is taken to name of the substituents in the alphabetical order when different alkyl group are present as substituents.
(iv) In the IUPAC name of an organic compound, the numbers are separated by comma from each other, das or hyphen (-) is put between a number and a letter and the successive words are merged into one word If the branched hydrocarbon contains more than one alkyl groups, then their names are not repeated, instead the number of the same alkyl substituents is written by prefix di for two, tri for three, tetra fo four, penta for five, hexa for six etc.
(v) When the organic compound has more than one alkyl group, then their names are written in the alphabetical order but the prefixed di, tri etc. are not considered for alphabetical order. Thus the correc name of the following compound is 3-ethyl-4, 4-dimethylheptane.
(vi) Numbering of Different Alkyl Groups at Equivalent Positions: When the two different alkyl groups are present at the equivalent positions, then the numbering of the parent chain is done in such a wa so as to assign the lower number of the alkyl group which comes first in the alphabetical order.
(vii) Cyclic Compounds: For naming cyclic hydrocarbons ‘cyclo’ prefix is used before the name of parent chain and the remaining rules are same as explained earlier.
Nomenclature of Unsaturated Hydrocarbons
(i) Select the longest possible carbon chain having maximum number of unsaturated carbon atoms or maximum number of double or triple bonds, even if the prior rules are violated.
(ii) Hydrocarbon containing both C=C and C☰C: When hydrocarbon contains one double bond and one triple bond they are called alkenynes (not alkynenes). The parent chain is numbered in such a way that multiple bond (double or triple) is assigned the lowest possible number. When these bonds are located at equivalent positions, the double bond is given priority over the triple bond.
Nomenclature of Organic Compounds having functional group
Functional Group: Functional group may be defined as an atom or a group of atoms bonded together in a unique manner present in a molecule which largely determines its chemical properties.
The presence of a functional group is indicated by either adding their suffixes or prefixes. The prefixes and suffixes of same functional groups are given in the following table.
The organic compounds with same functional group show similar chemical properties. For example, alcohols like CH3OH, CH3CH2OH,(CH3)2CHOH, etc, all produce hydrogen when treated with sodium metal.
- Identify functional groups.
- Decide the principal functional group according to its position in priority list. The principal functional group is identified by suffix and the other functional group by prefixes.
- Select the longest chain containing the functional groups including C=C or C☰C bond or both as the main chain and name the hydrocarbon on the basis of number of carbons in the main chain.
- Derive from the hydrocarbon name the parent name of the compound for the principal functional group.
- Assign number of carbon atoms in the main chain so that the principal functional group is given the lowest possible number.
- Put the positional number for the functional group, if necessary in the parent name at suitable place.
- Complete the name of the compound by placing substituents just before the parent name.
Nomenclature of Substituted Benzene Compounds
(1) For naming the substituted benzene compounds, the prefix used for the substituent is prefixed to the word ‘benzene’ simply.
(2) Many substituted benzene compounds are universally known by their common names.
Isomerism
Such organic compounds which have the same molecular formula but differ from each other in their properties are called as isomers and the phenomenon is called as isomerism. It is of two types:
- Structural isomerism
- Stereoisomerism
1. Structural Isomerism: Compounds having the same molecular formula but different structure are called structural isomers and the phenomenon is called structural isomerism. It is also known as constitutional isomerism. It is of the following types:
(i) Chain Isomerism: The compounds having same molecular formula but different chain of carbon atom. For example: Butane and 2-methyl propane are chain isomers.
(ii) Position Isomerism: Compounds having the same molecular formula but different in position substituents, C = C, C ≡ C or functional group are called position isomers.
(iii) Functional Isomerism: The compounds having same molecular formula but different functional groups in the molecule are called functional isomers.
(iv) Metamerism: The compounds having same molecular formula but different alkyl group on either side of the functional group, are called metamers.
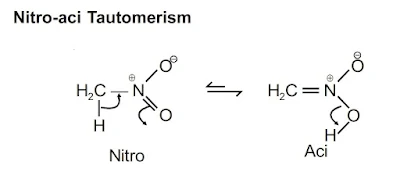
(v) Tautomerism: When two or more constitutionally distinct compounds are in dynamic equilibrium because of the shift of an atom (generally proton) from one place to another place in a molecule. The phenomenon is called Tautomerism and various constitutional isomers are known as tautomers.
2. Stereoisomerism: Isomers which have the same structural formula but have different relative arrangement of atoms or groups in space are called stereoisomers and the phenomenon is called stereoisomerism. It has three types:
- Geometrical Isomerism
- Conformational Isomerism
- Optical Isomerism
(i) Geometrical Isomerism: When two compounds differ in spatial arrangement of groups because of restricted rotation, these compounds are known as geometrical isomers.
Fundamental Concepts in Organic Reaction Mechanism
A. Fission of a Covalent Bond
Organic reactions usually involve making and breaking of covalent bonds. The fission of bonds can take place in two ways:
(i) Heterolytic Fission: When a covalent bond between two atoms A & B breaks in such a way that both the electrons of the covalent bond are taken away by one of the bonded atoms, the mode of bond cleavage is called heterolytic fission. Heterolytic fission is usually indicated by a curved arrow (↷) which denotes a two-electron displacement.
(ii) Homolytic Fission: If a covalent bond breaks in such a way that each atom takes away one electron of the shared pair, it is called homolytic or symmetrical fission. Homolytic fission is usually indicated by a fish arrow (↶↷) which denotes a one-electron displacement.
B. Attacking Reagents
(i) Electrophiles: A reagent that takes away an electron pair is called electrophile. There are positively charged or neutral species which are deficient of electrons and can accept a pair of electrons. They are also called electron-loving (philic) or electron-seeking species (E). Ex- H+, H3O+, Cl+, CH3+, NO2+, AlCl3, BF3 etc.
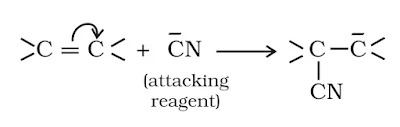
(ii) Nucleophiles: A reagent that brings an electron pair is called nucleophile. These reagent contain an atom having unshared or lone pair of electrons. A nucleophile is electron-rich and seeks electron-deficient sites i.e., nucleus-loving or nucleus-seeking (Nu). According to lewis concept of acids and basis, nucleophiles behave as lewis bases. Ex- X– OH–, CN–, RCOO–, NH3, H2O etc.
Electron Displacements in Covalent Bonds
(a) Inductive Effect (I-effect): This is a permanent effect which arises whenever an electron-withdrawing group is attached to the end of a carbon chain. To understand this, let us consider a chain of carbon atoms having Cl atom at one end.
C4-C3-C2-C1-Cl
Hence, Cl-atom is more electronegative than C so, the σ-electrons of the C—Cl -bond are attracted by or displaced towards the more electronegative atom. As a result, the atom Cl acquires a small negative charge (δ–) and C1 acquires a small positive charge (δ+) as shown below.
C4-C3-C2-C1δ+-Clδ-
(i) –I effect: If the substitutent attached to the end of the carbon chain is electron-withdrawing, the effect is called –I effect.
(ii) + I effect: If the substituent attached to the end of the carbon chain is electron-donating, the effect is called +I effect.
Applications
- Acidic and Basic strength of various organic acids and bases can be explained through this effect.
- Stability of carbocations and carbanions.
- Reactivity of alkyl halides.
- Diplole moment, bp, mp etc.
(b) Electromeric Effect (E-effect): It is temporary effect which operates in the organic compounds having multiple bonds i.e., double or triple bonds under the influence of an outside attacking species. As a result, one pi electron pair of the multiple bond gets completely transferred to one of the bonded atoms which is usually more electronegative.
The electromeric effect is shown by a curved arrow (↷) representing the electron transfer originating from the centre of the multiple bond and pointing towards one of the atoms which is more electronegative.
(i) +E effect: If the pi-electron pair of the multiple bond is transferred to the atom to which the attacking reagent gets attached, then the effect is called +E effect.
(ii) –E effect: In this case the pi-electron pair of the multiple bond is transferred away from the atom which gets linked to the attacking reagent, the effect is known as –E effect.
(c) Resonance or Mesomeric Effect (R-effect): The phenomenon of exhibiting more than one possible structure is called as resonance. The resonance can be explained clearly on the basis of structure of benzene. The benzene can be represented by following two canonical form.
(i) + R effect: If a conjugated system has an electron-donating group is said to have + R or + M effect. Such as –OH, –OR, –SH, –NH2, –NHR, –X (halogen) etc.
(ii) –R or –M Effect: If a conjugated system has an electron-withdrawing group is said to have – R or – M effect. Such as –CHO, –COOH, –COOR, –CN, –NO2, >C=O etc.
Aromaticity
Huckel's Rule of Aromaticity
Huckel’s rule is valid for compounds containing atleast:
- One planar ring (i.e., monocyclic)
- Conjugated (complete continuous conjugation) (c) Planarity
- (4n+2)π electrons where n is either zero or positive integer.
Hypercojugation
When an alkyl group is attached to an unsaturated system such as double bond or a benzene ring. The order of inductive effect is actually reversed. This effect is called hyperconjugation effect or Baker-Nathan effect.
Reaction Intermediates
The species produced during cleavage of bonds are called reaction intermediates, these are generally short-lived and highly reactive and hence cannot be isolated. The typical intermediates are:
(i) Carbocations: A carbocation may be defined as “A group of atoms with a positively charged carbon atom having six electrons in the valence shell after sharing”.
(ii) Carbanions: It may be defined as “chemical species bearing a negative charge on carbon and possessing eight electrons in its valence shell are called carbanions”.
(iii) Free Radicals: It may be defined as “an atom or group of atoms with an odd or unpaired electron.”
Types of Reactions
There are basically four types of reaction:
- Addition reaction
- Elimination reaction
- Substitution reaction
- Rearrangement
(i) Addition Reaction: In which a group adds to the system, unsaturated organic compounds comes under this category e.g.
CH2 = CH2 + H2 → CH3—CH3
(ii) Elimination Reaction: The reactions in which two atoms or groups of the molecule are removed are called elimination reactions.
CH3—CH2Br → CH2 = CH2 + HBr
(iii) Substitution Reaction: When a group is removed and another group takes its place. These can also be called displacement reactions. The displacement of the halide group by an OH group to form alcohol.
CH3Cl + KOH → CH3OH + KCl
(iv) Rearrangement: When the molecule rearranges itself by shifting its own part to some other site within the molecule. This is done to attain higher stability if it can be achieved. The various isomerization reactions come under this category.
CH3—CH2—CH2+ → CH3—CH+—CH3
Methods of Purification of Organic Compounds
The organic compounds whether isolated from a natural source or prepared in the laboratory are mostly impure. These are generally contaminated with some other substances. A number of methods are available for the purification. The choice of a particular technique or method depends upon the nature of the compound whether solid or liquid and also upon the nature of the impurities associated with it. The common techniques used for purification are as follows:
- Sublimation
- Crystallisation
- Distillation
- Differential extraction
- Chromatography
(i) Sublimation: Certain organic solids on heating directly change from solid to vapour state without passing through a liquid state. Such substances are called sublimable. This process is called sublimation. The vapours on cooling change back to the solid form. The sublimation process is used for the separation of sublimable volatile compounds such as camphor, naphthalene, anthracene, benzoic acid etc.
Solid ⇌ Vapour
(ii) Crystallisation: This is the most common method for purifying organic solids. This method is based on the differences in the solubility of the organic compound and its impurities in a suitable solvent.
(iii) Distillation: “Distillation is the process of converting a liquid into vapours upon heating and then cooling the vapours back to the liquid state”. The process of simple distillation is used to purify those organic liquids which are quite stable at their boiling points and the impurities present are non-volatile. Liquids such as benzene, toluene, ethanol, acetone, chloroform, carbon tetrachloride can be purified by simple distillation.
(iv) Differential Extraction: This technique is normally used to separate certain organic solids dissolved in water by shaking with a suitable organic solvent. The process called extraction is done in a separating funnel. The organic solvent selected should be such that (a) The given solid must be more soluble in the organic solvent than in water. (b) Water and organic solvent should not be miscible with each other.
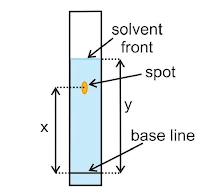
(v) Chromatography: Chromatography is a modern and sensitive techniques used for rapid and efficient separation or analysis of components of a mixture and purification of the compounds. “The technique of separating the components of a mixture in which separation is achieved by the differential movement of individual components through a stationary phase under the influence of a mobile phase”.
Qualitative Analysis of Organic Compounds
In the study of any organic compound, it is an important step to know the elements present in it. In addition to carbon and hydrogen, organic compounds contain some other elements, e.g., nitrogen, sulphur, halogens, etc. These are detected as follows:
(a) Detection of Carbon and Hydrogen: Carbon and hydrogen are detected by heating the compound with copper (II) oxide. Carbon present in the compound is oxidised to carbon dioxide and hydrogen to water vapours.
C + 2CuO ⟶ 2Cu + CO2
(b) Test for Nitrogen: About 2 ml of sodium extract is taken in a test tube and made alkaline by adding NaOH solution. To this reaction mixture is added freshly prepared FeSO4 solution and boiled for 3–4 minutes. The formation of Prussian blue colour or precipitate shows the presence of nitrogen.
Na + C + N ⟶ NaCN
(c) Test for Sulphur: If organic compound contains both nitrogen and sulphur, sodium thiocyanate is formed.
Na + C + N + S ⟶ NaSCN
(d) Test for Halogens: A portion of the sodium extract is Boiled with 2–3 ml concentrated HNO3 followed by cooling and addition of AgNO3 solution when a pale yellow precipitate partially soluble in ammonia solution indicates the presence of bromine.
NaBr + AgNO3 ⟶ AgBr + NaNO3
Summary
Condensed Structural Formula: The structural formulae obtained by omitting some or all the covalent bonds and by indicating the number of identical groups attached to an atom by subscript is called condensed structural formula.
Bond-line Structural Formula: In this formula of organic compounds, carbon and hydrogen atoms are not shown and line representing C–C bonds and drawn in zig-zag fashion. The only atoms specifically written are those that are neither nor hydrogen bonded to carbon.
Cyclic Compounds: These are compounds in which carbon atoms are joined in rings i.e., they are closed chain compounds.
Aromatic Compounds: Benzene and its derivatives are called aromatic compounds.
Functional group: Functional group is an atom or group of atoms or reactive part of the compound which determines physical and chemical properties of compounds.
Homologous Series : Homologous series is a series of compounds which has same functional group same general formula and show gradation in physical and chemical properties of compounds.
Isomerism: The phenomenon of existence of two or more compounds possessing the same formula but different structural formula and different physical and chemical properties are called isomerism.
Structural Isomerism: Compounds having the same molecular formula but different structures are classified as structural isomers. Chain Isomerism : The isomers, which differ in carbon atom chain, are called chain isomers and this phenomenon is called chain isomerisms.
Position Isomerism: The isomers, which differ in position of substituent or functional groups are called position isomers and this phenomenon is called position isomerism.
Functional Isomerism: Those isomers, which differ in functional groups are called functional isomers and this phenomenon is called functional isomerism.
Metamerism: Those isomers, which differ in alkyl group attached with the di or tri valent atom of functional group. These are called metamers and this phenomenon is called metamerism.
Stereoisomerism: Those compounds that have the same composition and sequence of covalent bond but differ in relative positions of their atoms or groups in space.
Free Radical: An atom or group of atoms containing odd unpaired electrons in excited state is known as free radical.
Carbonium Ion: An atom or group of atoms in which carbon atom has positive charge due to its incomplete octet is called carbonium ion.
Carbanion: An atom or group of atoms in which carbon atom carries a negative charge due to its complete octet is called carbanion.
Electrophile: It is positively charged or neutral species which is electron deficient.
Nucleophile: It is negatively charged or neutral species with lone pair of electrons.
Inductive effect: The permanent electron displacement along a chain due to the presence of polar covalent bond is known as inductive effect.
Electromeric Effect: This is temporary effect which involves the complete transference of the shared pair of electrons to either of the two atoms linked by multiple bonds on the demand of attacking reagent.
Resonance or Mesomerism: When a single structure fails to explain the known properties of a compound, a number of structures on the basis of the octet rule are given which explain most of the properties but not all the known properties of the compound. This phenomenon is called resonance or mesomerism.
Resonance Energy: It is equal to the difference between the energy of the resonance hybrid and of the most stable resonating structure.
Hyperconjugation: It is electronic interaction occurring between a double bond in a compound and a carbon hydrogen single bond in an attached alkyl group.
Substitution Reaction: A reaction in which an atom or group of atoms is replaced by some other atom or group of atoms without any change in the structure of the remaining part of the molecule.
Addition Reactions: When a molecule is added across a double or tripple bond to form single product called addition product, such reactions are called addition reaction.
Elimination Reactions: The reaction in which loss of at least two or more atoms or groups form a reactant molecule takes place with the formation of double or tripple bond are called elimination reactions.
Rearrangement Reactions: Those reactions in which there is shift in functional group or atom or group of atoms from one part of molecule to another position in the same molecule to form new molecule which is isomer of reactant molecule are called rearrangement reactions.
Sublimation: It is process of separating a volatile solid from non-volatile impurities, i.e., the substance which can change into vapours directly without becoming liquid on heating.
Fractional Crystallisation: It is process used for separating the constituents of a mixture of dissolved substance by making use of their differing solubilities in a solvent.
Steam Distillation: This method is used to separate organic compounds from non-volatile organic or inorganic impurities by distillation with steam.
Steam Volatile: The substance which changes into vapours by heating with steam.
Chromatography: It is process of separating, isolating, analysing and purifying complex mixtures. It is based on the principle of differential adsorption.