The p-Block Elements Class 11 Notes Chemistry Chapter 11
Introduction
The elements in which last electron enters into p-subshell are called as p-block elements. The number of p-orbitals is three and, therefore, the maximum number of electrons that can be accommodated in a set of p-orbitals is six, hence p-block contains six groups.
Boron Family
Group III A contains six elements : boron, aluminium, gallium, indium, thallium and ununtrium. The penultimate shell (next to the outermost) conains 1s2 in boron, 2s2 2p6 (8 electrons) in aluminium and (n–1)s2(n–1)p6(n–1)d10 (18 electrons) in other elements.
Boron is a non-metal and always form covalent bonds. Boron family is known as most heterogeneous family as there is no regular trend in all properties, as it comes after d-block, lanthanoid contraction, poor shielding of d-orbital, they have large deviation in properties.
I. Physical Properties
The atomic radius, ionic radius and density increases when one moves from top to bottom in a group in periodic table. While melting point decreases from B to Ga and then increases from (Ga to In). Ionisation energy decreases from B to Al, but shows a reverse trend in going from Al to Ga.
Read also: Organic Chemistry Some Basic Principles and Techniques Class 11 Notes Chemistry Chapter 12
II. Chemical Properties
1. Reaction with air: Impure boron in air forms oxide while pure boron is less reactive.
4B + 3O2 ⟶ 2B2O3
2. Reaction with water: Boron is not affected by water or steam under ordinary conditions. However, Aluminium reacts with cold water if oxide layer is not present on its surface.
4Tl + 2H2O + O2 ⟶ 4TlOH
3. Reaction with acids: Boron is not affected by non-oxidising acids like HCl and dilute H2SO4 while other elements dissolve and liberate H2 gas.
2Al + 6HCl ⟶ 2AlCl3 + 3H2
4. Reaction with alkalies: Boron, Aluminium, Gallium react with alkali solutions whereas Indium and Thallium are not affected by alkalies.
2B + 6NaOH ⟶ 2Na2BO3 + 3H2
Anomalous Properties of Boron
Boron, the first member of group 13 elements, shows anomalous behaviour and differ from rest of the members of its family. The main reason for this difference are :
- exceptionally small atomic and ionic size.
- high ionization enthalpy.
- absence of d orbital in its valence shell.
- It has higher melting and boiling point than those of the other members of its group.
Read also: Thermal Properties of Matter Class 11 Physics Notes Chapter 11
Compounds of Boron
[A]. Borax/Sodium Tetraborate (Na2B4O7·10H2O)
It is the most important compound of boron. It is a white crystalline solid. Borax dissolves in water to give an alkaline solution.
I. Preparation
From Boric acid: Boric acid is neutralised with sodium carbonate and the resulting solution is cooled to get crystals of borax.
H3BO3 + Na2CO3 ⟶ Na2B4O7 + H2O + CO2
II. Properties
(i) It gets hydrolysed with water to form an alkaline solution
Na2B4O7 + 7H2O ⟶ 2NaOH + H3BO3
(ii) Borax bead test: On heating borax first swells up due to elimination of water molecules. On further heating it melts to a liquid which then solidifies to a transparent glassy mass.
Na2B4O7.10H2O ⟶ Na2B4O7 + 10H2O
Na2B4O7 ⟶ 2NaBO2 + B2O3
(iii) It is a useful primary standard for titration against acids.
Na2[B4O5(OH)4]·8H2O + 2HCl ⟶ 2NaCl + 4 H3BO3 + 5H2O
[B]. Diborane : B2H6
The simplest boron hydride known, is diborane. It is prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
Read also: Conceptual Questions for Class 11 Physics Chapter 11 Thermal Properties of Matter
I. Preparation
3LiAlH4 + 4BCl3 ⟶ 3LiCl + 3AlCl3 + B2H6
II. Properties
(i) Stable at low temperature only, colourless and highly toxic.
(ii) B2H6 + 6H2O ⟶ 2H3BO3 + 6H2
(iii) B2H6 + 6Cl2 ⟶ 2BCl3 + 6HCl
(iv) B3H6 + 2Me3N ⟶ 2[Me3N.BH3]
Uses of Boron and Aluminium and Their Compounds
Boron Compounds
Boron is a hard solid having high melting point low density and very low electrical conductivity. Some important boron compounds are :
(a) Boron fibers: It is mixed with plastic to form a material which is lighter than aluminium but tougher and stiffer than steel hence it is used in body armour, missiles and aircrafts.
(b) Boron-10 (10B) isotope: Boron carbide rods or boron steel are used to control nuclear reactions as neutron absorbers.
5B10 + 0n1 ⟶ 5B11
(c) Borax: It is used in manufacture of enamels and glazes for pottery and tiles. It is also used in making optical glasses and also borosilicate glasses which is very resistant to heat and shock. It is used as an antispectic.
(d) Boric acid: It is used in glass industry, in food industry as preservative. It is also used as an antiseptic and eye wash under the name ‘boric lotion’. It is also used in manufacture of enamels and glazes for pottery.
(e) Boron carbide: Hardest boron compound.
I. Aluminium Compounds
Aluminium and its alloy are used in packing industry, utensil industry, aeroplane and transportation industry etc.
1. Alumina (Al2O3)
(a) Used in chromatography.
(b) Used in making bauxite bricks which are used for lining furnaces.
2. Aluminium chloride (AlCl3): Used in manufacture of dyes, drugs and perfumes and also in manufacture of gasoline. It is also used as catalyst in Friedel Craft reaction.
3. Potash Alum. [K2SO4⋅Al2(SO4)3⋅24 H2O]: Used in purification of water, leather tanning, as antiseptic and as a mordant.
Group 14 Elements : The Carbon Family
Group IV A contains six elements : carbon, silicon, germanium, tin, lead and ununquadium. The penultimate shell (prior to outermost) contains 1s2 -grouping in carbon, 2s22p6 (8 electrons) in silicon and (n–1)s2(n–1)p6(n–1)d10 (18 electrons) in other elements. This shows why carbon differs from silicon in some respects and these two differ from rest of the members of this group. General electronic configuration is ns2np2.
[A]. Atomic and Physical Properties
The important properties of carbon family are discussed below:
(1) Atomic Radii: The atomic radii of group 14 elements are less than the corresponding elements of group 13. However, the atomic radii increases down the family.
(2) Ionisation Energies: The higher ionisation energies than group 13 are due to the higher nuclear charge and smaller size of atoms of group 14 elements. While moving down the group, the ionisation energies decreases till Sn.
C > Si > Ge > Sn < Pb
(3) Oxidation state and valency: The elements of group 14 show tetravalency by sharing four of its valence electrons. Therefore, they have oxidation state of +4. In addition, Ge, Sn and Pb also show +2 oxidation state.
(4) Catenation: Catenation is ability of like atoms to link with one another through covalent bonds. Tendency decreases from C to Pb. It is due to the decreasing M-M single bond energy. Thus, the tendency for catenation decreases as:
C > Si > Ge > Sn > Pb
(5) Allotropy: All the elements of the carbon family with the exception of lead exhibit allotropy. Carbon exists as two important allotropic forms diamond and graphite.
[B]. Chemical Properties
1. Reactivity towards air: All members of this group form monoxide of the general formula MO such as CO, SiO, SnO and PbO. All members of this group form dioxides of molecular formula MO2 such as CO2, SiO2, GeO2, SnO2 and PbO2.
2. Reactivity towards water: In this family three members i.e., carbon, silicon and germanium are affected by water while lead is not affected by water due to formation of protective oxide film but tin decomposes with steam into tin dioxide and hydrogen gas.
3. Reactivity towards halogen: These elements form two types of hallides - MX2 and MX4. Most of the MX4 are covalent. SnF4 and PbF4 are ionic in nature.
Anomalous Behaviour of Carbon
Carbon shows anomalous behaviour due to its smaller size, higher electronegativity, higher ionization enthalpy and unavailability of d orbitals. Carbon atom forms double or triple bonds involving pπ-pπ bonding. Carbon has also the property to form closed chain compounds with O, S and N atoms as well as forming pπ-pπ multiple bonds with other elements particularly N, S and O. When we move down the group size increases and electronegativity decreases hence catenation tendency decreases. Order is
C >> Si > Ge ≈ S
Allotropes of Carbon
Carbon shows allotropism due to catenation and pπ-pπ bond formation. Carbon exists in two allotropic forms – crystalline and amorphous. The crystalline forms are diamond and graphite while the amorphous forms are coal, charcoal and lamp-black. The third form is fullerenes discovered by Kroto, Smalley and Curl.
Note: Tin has maximum number of allotropes.
Diamond
In diamond each carbon is joined to other four carbon tetrahedrally and carbon-carbon bond length is 1.54Å and bond angle is 109º28′ having sp3 hybridisation on each carbon. All four electrons in carbon are involved in bonding hence, it is bad conductor of electricity. Diamond is an excellent thermal conductor.
It is hardest natural substance known. It is transparent and has a specific gravity 3.52 and its refractive index is high (2.45). Difficult to break due to extented covalent bonding. Diamond is used for making cutters. Blades of diamond are used in eye surgery and as an abrasive for sharpening hard tools. Impure diamonds (black) are used in knives for cutting glass.
Graphite
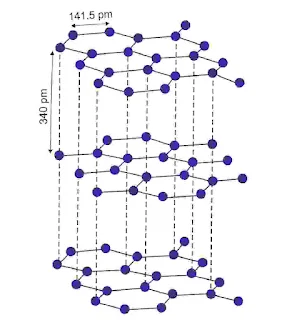
Each carbon is sp2 hybridised. It has layered structure. These layers are attracted by van der Waals force. Each carbon has one free electron in p-orbital, so it is a good conductor of electricity. All electrons get delocalized in one layer and form π-bond. Electron jumps from one orbital to another hence it is a good conductor of heat and electricity. In graphite carbon-carbon bond length is 141.5 pm and distance between adjacent graphite layer is 340 pm.
Graphite is used as a lubricant at high temperature. Oil gets burn or denatured at high temperature but graphite does not get denatured even at high temperature so, preferred over oil and grease.
Fullerene
It was made as a result of action of a laser beam or strong heating of a sample of graphite in presence of inert atmosphere. The sooty material mainly contains C60 with C70 (small amount). Most common fullerene is C60 called Buckminsterfullerene which has football-like structure. It contains 20 six-membered ring and 12 five-membered ring. It is used to make ball bearings.
Coal
It is the crude form of carbon. It has been formed in nature as a result of slow decomposition of vegetable matter under the influence of heat, pessure and limited supply of air. The successive stages of transformation are : peat, lignite, bituminous, steam coal and anthracite. Bituminous is hard stone, burns with smoky flame. The superior quality is anthracite which burns with non-smoky flame.
Uses of carbon
- Graphite: In making lead pencils, electrodes of electric furnances, as a moderator in nuclear reactor, as a lubricant in machinery.
- Charcol: In removing offensive odour from air, in removing fused oil from crude spirit, in decolourising sugar syrup, in gas masks etc.
- Carbon black: For making printing inks, black paints, Indian inks, boot polishes and ribbons of typewriters.
- Coal: For the manufacture of coal gas, coal tar, coke and synthetic petrol.
Compounds of Carbon
(1) Carbon Monoxide (CO)
Preparation: Carbon monoxide is majorly prepared by
2C + O2 ⟶ 2CO
Properties:
(i) Burns with blue flame
2CO + O2 ⟶ 2CO2
(ii) CO + Cl2 ⟶ COCl2 (Phosgene)
(iii) CO + 2H2 ⟶ CH3OH
(iv) Many of the transition metals form metal carbonyls
Ni + 4CO ⟶ Ni(CO)4
(2) Carbon Dioxide (CO2)
Preparation: Carbon dioxide is mostly prepared by decomposition of carbonates and bicarbonates
- (i) CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2
- (ii) CaCO3 ⟶ CaO + CO2
Properties: Carbon dioxide is an acidic, colourless gas. The important properties are:
- (i) Zn + CO2 ⟶ ZnO + CO
- (ii) 2Mg + CO2 ⟶ 2MgO + C
- (iii) 2NaOH + CO2 ⟶ Na2CO3 + H2O
- (iv) Na2CO3 + H2O + CO2 ⟶ 2NaHCO3
Summary
Allotropes: Those compounds which have different physical properties but similar chemical properties are called allotropes e.g., diamond and graphite.
Catenation: Tendency of carbon atom to link with itself to form long chain is called catenation.
Inert pair effect: Decrease in tendency of ns2 electron pair to participate in bond formation with increase in atomic number is called inert pair effect.
Silicones: It is organosilicon compound containing repeated R2SiO units.
Silicates: Compounds in which anions are either discrete SiO4-4 units or a number of such units combine together through corners.
Alums: All double sulphates having one monovalent basic radical and one trivalent basic radical.
Boranes: Hydrides of boron are called as boranes.